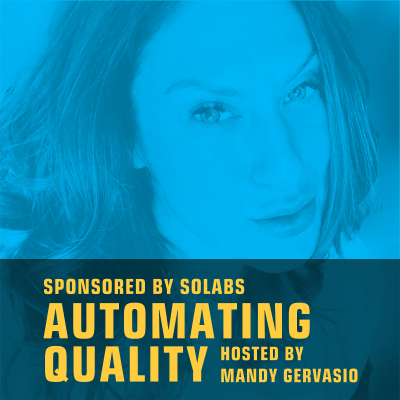
Automating Quality
Podcast de SOLABS, Mandy Gervasio, Philippe Gaudreau, and Guests
Empieza 7 días de prueba
$99 / mes después de la prueba.Cancela cuando quieras.
Valorado con 4,7 en la App Store
Acerca de Automating Quality
Welcome to the Automating Quality show sponsored by SOLABS, with your host Mandy Gervasio, Technology and Life Sciences industry veteran. The Automating Quality podcast is designed to provide professionals in the regulated Life Sciences industry with best practice perspectives as well as employable strategies and tools relevant to current industry trends. Listeners will come to understand pressing issues in the space and hear best in class thought leadership on various topics such as Quality, Training and Regulatory Compliance driven from an automation lens.
Todos los episodios
62 episodiosWelcome to Automating Quality, the life sciences–focused show that bridges the gap between automation and quality management. In today’s episode, our host Philippe welcomes Jim Ferguson, President and Owner of NuQual Consulting. With over 25 years of experience in the life sciences industry, Jim brings deep expertise in supplier quality and compliance. Together, they dive into the world of internal audits — how they can be used not only to maintain compliance but also to proactively identify and resolve potential observations before a regulatory inspection takes place. Key Takeaways 01:22 Introducing today’s guest, Jim Ferguson from NuQual Consulting 02:18 What quality-focused events has Jim been attending lately? 04:30 How can internal audits help organizations prepare for regulatory inspections or other types of audits? 07:25 What is the value of outsourcing internal audits? 08:35 How can companies ensure that audit findings actually lead to meaningful changes? 12:57 What does a strong response to internal audit findings look like? 17:30 What are some common pitfalls organizations tend to overlook during internal audits? Contact Jim at https://nuqualconsulting.ca/ [https://nuqualconsulting.ca/] Contact us at solabs-podcast@solabs.com
Welcome to Automating Quality, the life sciences–focused show that bridges the gap between automation and quality management. In today’s episode, Ed and Philippe explore how quality can bring added value to a company and discuss strategies to shift organizational mindsets to unlock the full potential of quality departments. Ed Siurek brings over 30 years of hands-on experience in applying quality standards and ensuring regulatory compliance in the pharmaceutical and medical device industries. He has trained hundreds of quality professionals and is a strong advocate for positioning quality as a driver of business value. After 13 years with a consulting firm, Ed recently transitioned full-time to his own company, ES3 Solutions Inc. Key Takeaways 00:53 – Introducing Ed Siurek 02:20 – Why is quality sometimes perceived as a cost? 05:50 – Always remember: our products are ultimately used on people 09:02 – “If it’s not written down, it didn’t happen” 10:20 – Use your risk report — don’t let it collect dust 13:20 – How are regulatory bodies viewed in the industry today? 21:15 – What is the role of a quality plan? Contact us at solabs-podcast@solabs.com [solabs-podcast@solabs.com] for questions or suggestions. Contact Ed Siurek at Always improving ES3 Solutions [http://www.es3solutions.com/]
Welcome to Automating Quality, the life sciences-centric show that bridges the gap between automation and quality management. In today’s episode, Michael and Philippe discuss investigation methods, how to apply them in detail, and the various tools available for conducting investigations. Michael Tyo has worked in the biotech industry for 45 years, from engineering roles to quality assurance. Michael is now consulting at Tyo Biotechnology Consulting. Key Takeaway 01:24 Introducing today’s guest: Michael Tyo 01:55 Today’s topic is investigative methods and what happens when things go wrong 03:36 The Fishbone method 06:28 Why human error cannot be a root cause? 07:55 The 5 Whys method 09:35 What needs to be included in the investigation report? 11:30 What does the FDA expect from investigations? 12:38 Lessons from recent FDA’s warning letters Contact Michael at mtyo@tyobio.com [mtyo@tyobio.com], Tyo Biotechnology Consulting [http://www.tyobio.com/] Contact solabs at solabs-podcast@solabs.com [solabs-podcast@solabs.com]
Automating Quality Episode 59 – Inspection Readiness with Michael Tyo Welcome to Automating Quality, the Life sciences-centric show that bridges the gap between automation and quality. In today’s episode, we discuss inspection readiness, from why we are doing them to how to conduct investigations properly. Michael Tyo has worked in the biotech industry for 45 years, from engineering roles to quality assurance. Michael is now consulting at Tyo Biotechnology Consulting. Key Takeaways 00:42 Introducing today’s guest: Michael Tyo 01:42 Today’s subject is the investigations, from why they exist to how they must be performed 02:22 Why are we doing investigations? 04:21 What may trigger an investigation? 08:04 What must be investigated? 10:00 Who is responsible for doing investigations? 14:41 What are the tasks once the investigation is launched? Contact Michael at mtyo@tyobio.com [mtyo@tyobio.com], Tyo Biotechnology Consulting [http://www.tyobio.com/] Contact solabs at solabs-podcast@solabs.com
Welcome to Automating Quality, the life sciences-centric show that bridges the gap between automation and quality management systems. This episode is the second in a two-part series discussing the Software Bill of Materials (SBOM) with guest Joseph Silvia. In this episode, we discuss the definition of AIBOM, how it differs from SBOM, and take an educated guess at their future. Joseph is the CEO of MedWareCyber, a consulting firm specializing in FDA readiness, cybersecurity, and software readiness for the medical devices industry. He is extremely knowledgeable about the regulatory landscape, and we frequently refer to him for his regulatory expertise. Key Takeaways; 01:05 Introducing guest Joseph Silvia 02:20 What is the concept of AIBOM? 04:23 Why AIBOM and SBOM should be separate discussions 07:45 How does open-source software impact those bills of material 11:22 How do you assess the risk of an AIBOM? Contact us at solabs-podcast@solabs.com

Valorado con 4,7 en la App Store
Empieza 7 días de prueba
$99 / mes después de la prueba.Cancela cuando quieras.
Podcasts exclusivos
Sin anuncios
Podcast gratuitos
Audiolibros
20 horas / mes























